Deloitte - A look at those about to redefine Novel Food Regulations
- Feb 17, 2023
- 9 min read
An opinion piece from The Hemp Hound Agency

On 15/02/2023 I spend most of the morning trying to find out who had won the tender for an independent review of Novel Foods Regulations.
For some reason, it didn't appear until the afternoon, despite the fact that the contract started on 12/12/2022. What's more is that the FSA as of yesterday (16/02/2023) have only just announced the winner to the public, 12 days before the report is supposed to be submitted back to them.
So the question is, who is Deloitte, and what baggage do they come with?
Well in 2011, co-founder of GW Pharmaceuticals Geoffrey Guy was the recipient of the Deloitte 'Director of the Year Award' in Pharmaceuticals and Healthcare, so we're already on interesting ground.
Now I'm not implying that there is anything wrong here, but just so Deloitte know, the last time the UK hemp and CBD industry came across undeclared interests was when it came to light that a member of the board for the Centre for Toxicity, which endorsed 16 lab reports from GW Pharma to 'regulate' Novel Foods for CBD products, was from Reading University, who had awarded Geoffrey Guy an honorary doctorate in science 3 years before Novel Foods properly began (one year if we go with the Novel Foods false start in 2017), and had received a fair chunk of money for R&D into cannabinoids various by GW.
www.reading.ac.uk/news-archive/press-releases/pr681951.html
The question is, did Geoffrey Guy win that award because GW were clients of Deloitte in 2011, and if so, are they now? And regardless of which is the case, does that constitute a conflict of interest that should have been reported before any contract was signed?
I'm just going to state the obvious here for a second
Check out Deloitte online, who should I feel more worried about, them, or who may or may not be their clients, at some time or another?
It's a fair comment as they had revenue of $59.3 billion in 2022, and they have other clients that have interests within the hemp and CBD industry, but let's just go with who we know first.
www.sec.gov/Archives/edgar/data/1232524/000119312522061827/d164239dex233.htm
Filing date is just over 2 months after Jazz Pharmaceuticals brought GW Pharmaceuticals
So GW, Jazz and Deloitte under one roof, a directors award from 2011 for Mr Guy, and now we come to these files below, which show that Deloitte have been working with Associated British Foods, who own British Sugar, who in turn supply GW/Jazz Pharmaceuticals with CBD rich biomass for their products.
Word search for Deloitte, they are named 5 times in small print
If this was a game of poker and I had those cards in my hand, I'd be going all in!!!
Seriously though, that's too much before we even look into Deloitte 'the company', but before I do, lets just go back to their client list. The two that jump out are Proctor and Gamble, and GlaxoSmithKline, both have connections to CBD and cannabinoid interests, and both I believe were named as industry stakeholders in 2019 when the EU hemp and CBD industry met at Brussels to discuss Novel Foods for CBD products.
Then of course, there's Monsanto, who also have interests with cannabis, and that scares the living hell out of me!
Further to that we have Pfizer, who I can't find listed as a client of Deloitte but clicking here will show there's a link between the two, and of course Pfizer have just stepped into the medicinal cannabis sector with a big buy out of Arena Pharmaceuticals.
So, do Deloitte have too many interests at hand to be in a position to offer their opinion on the Novel Foods regulations? I'd say yes, and that's before we touch on the screenshot below
To the left is an online document from Jazz Pharmaceuticals, submitted to the EU approximately 7 months after they had brought GW Pharmaceuticals. It's also from the same timeframe where Deloitte, Jazz and GW are mentioned within the same 'Consent of Independent Registered Public Accounting Firm' document above.
At the centre of the fields of interest section is 'Food safety', but we know that their version of this comes from judging food supplemental products by lab reports for pharmaceutical extracts, so I feel it's safe to say, especially in light of what is happening in the US with the FDA, that GW/Jazz's interests are more in redefining what is a food, so that their shareholders profits are safe.
How better to do that than to get someone you know, or that you're a client of, to redefine Novel Foods regulations? I mean it wouldn't be the first time GW have asked someone to do something for them: the MHRA in 2016 to redefine CBD as a medicinal compound, The HO to ask FSA to define all cannabinoids as Novel Foods in 2017, the HO to the VMD in 2018 to outlaw CBD products for pets, and I'm pretty certain that getting the HO to question controlled cannabinoid levels and testing requirements in 2021 was them as well.
Of course the HO, MHRA, FSA, GW/Jazz and anyone else mentioned in this article are more than welcome to tell me where I've gone wrong, but considering most are too hesitant to reply to Freedom of Information requests on topics in this area, I don't think they will!
Let's just rewind to that part where I mentioned the government contract details for this review of Novel Foods regulations weren't available until yesterday afternoon.
The weird thing about this to me is that the FSA had replied to my public interest disclosure complaint the day before I discovered Deloitte had won the contract to review Novel Foods regulations, and two days before officially announcing it.
A standard reply is supposed to take 20 working days, 40 if it's more complex, but the FSA stated they might not reply fully until the end of March, which would be 55 working days.

If you would like to know what the extra complaint is, click here. It's about the FSA not telling us what the rules are, and it also relates to the ACI, who I believe have been part funded by GW/Jazz throughout their existence, although if you look at their staff list on LinkedIn, you'll see that this might just be about to change
After receiving this, I found myself wondering what was happening between the 7th of February when the FSA said they would reply to my complaint, until the 31st of March, which happens to be the 2nd anniversary of when Novel Foods applications were to be submitted by.
I remembered that the Novel Foods regulations review contract was out for tender, I spend hours searching for it, but the file only appeared after lunch, and after I used the same word search as I had before. 24 hours after it 'appeared' online, the FSA announce a contract that could effect us all, 12 days before it's due to be completed.
I hate to say this but I have concerns that the FSA had been hiding that contract in the hope that I wouldn't find it, and that is due to me making a public declaration that I would do background checks on anyone who was awarded it.
Conspiracy?
Well I don't want to appear to be up my own behind, but let's just compare this to the timeline of my complaint against the FSA's handling of Novel Foods:
The complaint was lodged on 15/07/2022, I received a request to refine that complaint the next month (which is above), I was asked to further refine it to which I questioned whether the FSA were following the Civil Servants code, and then I was offered an interview on 10/10/2022.
Now to my understanding, the tender for the Novel Foods Regulatory Framework Review was first placed online on 03/10/2022, one week before my meeting with the FSA, but a month after I had quoted the Civil Servants Code. It was awarded on 19/12/2022, removed from the government tender list and replaced 1 day after I received the FSA's response stating that they need more time.
It instantly reminds me of that meeting with the FSA on 10/10/2022
Upon raising the issues of GW's influence over the hemp and CBD industry, Dr James Cooper who participated stated "There's no conspiracy here you know!", but actually he's very wrong, or he was trying to mislead me. You don't need a shiny tinfoil hat or 16 lab reports to see that GW/Jazz's influence over Novel Foods is being allowed.
Enough about GW/Jazz though, lets look back at Deloitte
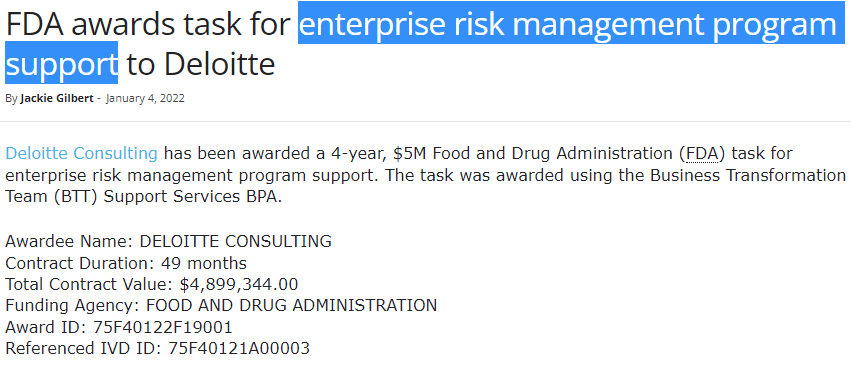
And wouldn't you know it, it seems that they've had a few contracts with the FDA, who on 26/01/2023 announced that they are looking for political intervention over CBD products, and that they had been looking at lab reports from GW/Jazz for Epidiolex to determine the 'safety' of CBD products.
Awarded on 04/01/2022 - The question is, whose risk are they managing?
So Deloitte have clients whose interests lie in CBD being defined as a medicine, and are now working with the FDA, and the FSA. If you look here, you'll also see that they have some sort of relationship with the Home Office as well, who themselves have been guiding GW/Jazz over a 20+ year working relationship, as well as maintaining the UK's "Skunk is bad" narrative to further protect their interests.
Needless to say, if I were to 'go missing', all of the names above need to be investigated!
Why though, because we all know there is a way the food supplemental and pharmaceutical industries to co-exist, right?
It's simple, we have health claims, they have medical claims. We sell whole-plant products, they sell refined cannabinoid isolated products. We don't go over 15/20%, and they can have the insanely large window that still leaves them for medicinal access, so what's the problem?
Well I hate to say it, but it's not all about CBD
Whilst we always get dragged back to the 16 lab reports for the extract used to create Epidiolex, we've also got to look into GW's 148 patents. Over 50 (I believe) name CBD, but over 35 of them name THC D9, whilst others include other cannabinoids including the 12 named in the UK as Controlled.

GW/Jazz want the whole-plant, so that no-one gets access to these or any other cannabinoids that is being, or could be researched. and whilst we're here I suppose I best give you a heads up on the 3 cannabinoids that will be gracing this lists soon: CBDa, CBG, and CBC, as well as all the precursors to them.
If you want confirmation of this, you might want to check out the Ferra Labs contract that I found myself looking at whilst I was trying to find the one for the Novel foods regulation review. You'll see CBDa, CBG and CBC mentioned specifically in amongst a list that wasn't 'limited too', with references to the ACMD report submitted to the HO in 2021 which also points out CBG and CBC by name.
You also need to know that the contract with Ferra Lab works on the basis of them reporting companies who breach the ACMD directions, which I feel indicates that we're no longer working by MoDR 2001 Section 2, despite there being no change in the law to reflect this.
See section 2.0 on p.5 for named cannabinoids, see section 3. 2. 3 on p.7 to show that ACMD advice is being followed, and then click here for the ACMD advice and scroll down to point 5 specifically. Also, ask yourself why a lot of the Ferra Labs contract is redacted...
And when you see that, ask yourself how they could be awarded over £160,000 to test 100 products!
Deloitte's and Ferra Labs contracts finish on 28/02/2023 and 31/03/2023 respectively, so there's two reasons why I feel my complaint has been 'delayed'.
Past there though, this is all about Deloitte's suitability to conduct the review into Novel Foods regulations, and I'm sorry to say this but it feels like they have been placed into that contract, and I believe that it is because an 'independent body' can best serve the needs of their clients when it's hidden up until the last moments that they are in fact about to change Novel Foods regulations, knowing full well that the only ones truly effected are those who are an inconvenient competition to GW's dominance in the medicinal CBD and cannabinoid industry, which is basically the hemp and CBD food supplemental industry.
And to all of those who'll say "it's a review of Novel Foods Regulations, it says nothing about CBD!"
That's very true, but it doesn't have to say that does it. Look into Deloitte, trust me, there's a lot more to find in regards to them, their clients and cannabis. Then ask why a company like this would be happy to take £130,000 to help redefine Novel Foods, when to be perfectly honest, that's peanuts to them!
Also ask yourself why there's a need to redefine 'not novel' foods, as well as what can qualify for an Article 4 submission.
Yep, that's right, cold pressed hemp oil is on the chopping block too!
And all of that may well be coming from Deloitte, who's recently been highlighted by the Financial Times as having no expertise in the areas that they advise in, but if you're still unconvinced, allow me to share one golden rule I discovered early whilst in this industry. When you see an update on GW/Jazz, government agencies like FSA, MHRA, or the US' FDA tend to make supporting announcements, so we really need to pay attention to yesterdays update that shows GW/Jazz supplying a worldwide study on CBD vs psychosis.
For any questions or information about The Hemp Hound Agency, email cefyn.jones@hemphound.co.uk







Invaluable as ever Cefyn. Thanks for releasing your continuing research and discovery in an easy to digest format. It feels very much like the dots are too well aligned. It's looking shockingly like they'll all be joined up shortly. Shameful, sad times for the UK.